- Home
- World
- News
- Pfizer Applies for COVID-19 Vaccine Use Authorization in S. Korea - Drug Safety Minister
Pfizer Applies For COVID-19 Vaccine Use Authorization In S. Korea - Drug Safety Minister
Umer Jamshaid Published January 25, 2021 | 04:32 PM

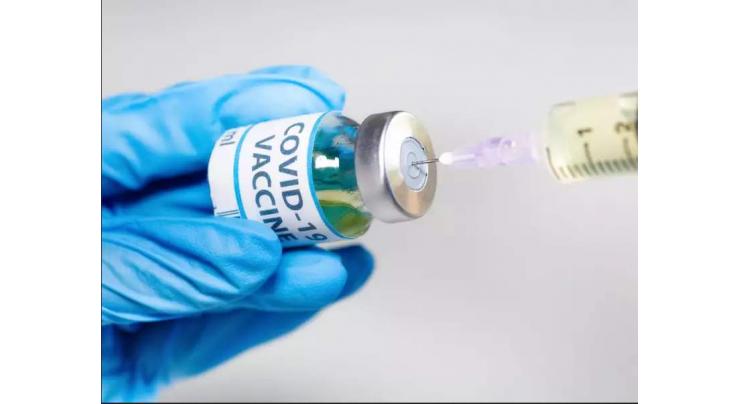
US pharmaceutical giant Pfizer has applied for the authorized use of its COVID-19 vaccine in South Korea, Minister of Food and Drug Safety Kim Gang-lip said on Monday
SEOUL (UrduPoint News / Sputnik - 25th January, 2021) US pharmaceutical giant Pfizer has applied for the authorized use of its COVID-19 vaccine in South Korea, Minister of food and Drug Safety Kim Gang-lip said on Monday.
On December 23, the East Asian country signed a contract with Pfizer to receive 20 million doses of its vaccine. Deliveries will start in the third quarter of 2021 and should be completed in full by the end of the year.
"This afternoon, Pfizer applied for use authorization of the vaccine. We will thoroughly review the safety and effectiveness of the product," Kim told reporters.
South Korea is set to begin the vaccine rollout in February, hoping to achieve herd immunity by November.
Along with the Pfizer/BioNTech vaccine and those provided via the World Health Organization's COVAX Facility, the country will also receive vaccines from Johnson & Johnson, Janssen, Moderna and AstraZeneca/Oxford.
Frontline health workers and care home staff will get the shots in the first quarter of the year, followed by the other medical personnel and those aged 65 and older in the second quarter. Those suffering from chronic diseases and adults aged between 19-64 will be immunized in the third quarter.
Recent Stories

Currency Rate In Pakistan - Dollar, Euro, Pound, Riyal Rates On 25 April 2024

Today Gold Rate in Pakistan 25 April 2024

Mired in crisis, Boeing reports another loss

Session Awarding Ceremony 2024 held at Cadet College Muzaffarabad

Austrian ski great Hirscher to make comeback under Dutch flag

Pakistan, Japan agrees to convene 'Economic Policy Dialogue'

FM Dar conveys deepest sympathy on torrential rains devastation in UAE

Spain PM Sanchez says weighing resignation after wife's graft probe

Tennis: ATP/WTA Madrid Open results - 1st update

Long-lost Klimt portrait auctioned off for 30 mn euros

Osaka seals first win on clay since 2022 in Madrid

Earthquake jolts Karachi
More Stories From World
-
Star Dudamel brings inclusive vision to New York Philharmonic
20 minutes ago -
Paris dream of swimming in the Seine finally within reach
49 minutes ago -
Portugal's Carnation Revolution, 50 years on
50 minutes ago -
Tough times for Argentine factories as consumers penny-pinch
59 minutes ago -
Use of alcohol and e-cigarettes among youth 'alarming': WHO
60 minutes ago -
Football: Italian Cup result
1 hour ago
-
Bird flu in humans? Experts see little risk
1 hour ago -
Car giants vie for EV crown at Beijing's Auto China show
1 hour ago -
Meta profits soar but costs of AI cause worry
1 hour ago -
Tennis: ATP/WTA Madrid Open results - collated
2 hours ago -
Football: French Ligue 1 results - collated
2 hours ago -
Meta sees profits soar in first quarter
2 hours ago